HETEROTOPIC BONE FORMATION IN PEDIATRIC PATIENTS
CLINTON F. PICKETT D.O., Orthopaedic Resident
FREEMAN MILLER M.D., Attending Pediatric Orthopaedic Surgeon
RICHARD KRUSE D.O., Attending Pediatric Orthopaedic Surgeon
May 9, 1996
CLINICAL CASE PRESENTATION
ORTHOPAEDIC DEPARTMENT
THE ALFRED I. DUPONT INSTITUTE
WILMINGTON, DELAWARE
CASE HISTORY:
A nine year-old male with a diagnosis of cerebral palsy spastic quadriplegia
presented for a follow-up exam. This patient was non-ambulatory and non-verbal.
Patient had good head control and was capable of sitting if propped up.
There was a wind-blown posture with the left hip being adducted and subluxated
30- 40 percent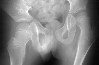 .
On 9\95 patient underwent bilateral release of iliopsoas, gracilis, adductor
longus, distal hamstrings and right tensor facia lata release. Four weeks
later the patient still required valium and analgesics due to pain and
muscle spasms. At that time a firm mass was palpated in the left groin.
On 11\95 The patient was still having pain
.
On 9\95 patient underwent bilateral release of iliopsoas, gracilis, adductor
longus, distal hamstrings and right tensor facia lata release. Four weeks
later the patient still required valium and analgesics due to pain and
muscle spasms. At that time a firm mass was palpated in the left groin.
On 11\95 The patient was still having pain 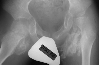 .
Four months post-op the patient was able to start aquatic therapy. Seven
months post-op the patient had increased range of motion with less pain.
.
Four months post-op the patient was able to start aquatic therapy. Seven
months post-op the patient had increased range of motion with less pain.
HETEROTOPIC BONE FORMATION:
Heterotopic bone formation was first described in 1692 when Guy Patin
described myositis ossificans progressiva in children.(1) Better understanding
and descriptions came about and in 1918 Dejerine and Ceillier described
heterotopic ossification as a complication of intermedullary gunshot wounds
during World War I(2) . They postulated the etiologies of local edema and
a neurogenic factor . In 1961 Damanski associated improved post-trauma
care with decreased incidence of heterotopic ossification(3). Descriptions
of HO in children have been described following ilio-psoas release(11,12,13),
posterior rhizotomy and femoral osteotomy (10), spinal cord injury(4),
and spinal fusion (4,12) in recent literature.
Confusion surrounds the subject of heterotopic ossification regarding
definition, etiology, incidence, and treatment. Heterotopic ossification(HO)
is the formation of lamellar bone ( which may mature with time) where bone
does not usually form in soft tissues. Myositis ossificans is a condition
in which HO occurs in muscles and other soft tissues(21). There are three
types of myositis ossificans circumscripta, myositis ossificans progressiva,
and localized traumatic myositis ossificans (21).Ectopic calcification
is mineralization of soft tissue structures usually due to physical or
chemical trauma such as calcific tendonitis-histologically the deposits
are not bone forming(4).
ETIOLOGIES:
Etiologies of HO include trauma , neurogenic, and myositis ossificans
progressiva. Traumatic etiologies include after spinal fusions , total
hip arthroplasty, ORIF of acetabular fractures, soft tissue releases about
the hips and burns. Neurogenic etiologies include closed head trauma, spinal
cord injury, CNS infections, tumors, strokes, tetnus, polio, tabes dorsalis,
multiple sclerosis, and following a selective posterior rhizotomy. Myositis
ossificans progressiva is a rare autosomal dominant disease which begins
in early infancy which initially involves the muscles of the back, neck,
and shoulders and then progresses to immobilize the patient in early life(4).
PATHOPHYSIOLOGY:
Pathophysiology of HO is unclear. Histologic studies of heterotopic
bone reveal the percentage of osteoblasts is typically double that of normal
bone indicating that the bone being formed is metabolically active(14).
One distinguishing feature is that the new bone and mature bone lacks periosteum(15).
Heterotopic bone is often diffuse and does not always follow anatomic planes
of tissue. Bone morphogenic protein is a potential inducer of undifferentiated
mesenchymal cells which are precursors of cartilage and bone forming elements.
It has been theorized that BMP may induce inappropriate differentiation
of pluripotential cells, mesenchymal cells or fibroblasts into osteoprogenitor
cells(5). Fujimori et al(6) studied the role of BMP and interleukin-1 in
a rat model which had collagen induced arthritis . Non-osteogenic cells
such as transitional epithelial cells from the urinary bladder(7), various
sarcoma viruses, and t-lymphocyte mitogens have been associated with HO(7,15).
The possible associatiion of prostaglandin E2 with heterotopic ossification
has been studied in a rat burn model(8).
EVALUATION:
Evaluation of a patient includes an adequate physical exam. The initial
clinical finding is decreased range of motion. There may be localized tissue
hyperemia, swelling and tenderness to palpation. Initial onset is usually
4-16 weeks but ranges from two to fifty-two weeks(14). In addition to pain
there may be increased spasticity of the limb. Differential diagnosis is
infection(14,9),tumor, thombophlebitis(14,9),reflex sympathetic dystrophy,
and many patients will have multiple and complex problems(14). Traumatic
type of HO is found near focus of trauma. Neurogenic type of HO is found
below the level of spinal cord injury usually on the side of spastic muscles.
XRAYS:
Radiographic findings of HO on plain films is evident at 4-6 weeks,
usually trabeculation is absent. Payne and DeLuca(10) define HO in soft
tissues as radiodensity 5 mm from the femoral shaft and not initially adjacent
to the femur which is periosteal callus and HO is usually a flocculent
pattern.Technetium three-phase bone scan is positive in the initial phase
of HO and is 90% sensitive in 2-4 weeks after injury. Return to baseline
is 7-12 months after injury(14). Bone scans may be used to assess the level
of maturity of the HO. Computed tomography can differentiate native vs.
ectopic bone by revealing the osseous architecture(14).Computed tomography
may also be useful in planning surgical approach. Magnetic resonance imaging
can demonstrate soft tissue swelling but receives only limited signal from
calcified tissue(14). Ultrasound has been shown to detect HO earlier than
plain radiographs in eight consecutive patients by Thomas and Amstutz (17).
LABS:
Laboratory findings include changes in serum levels of alkaline phosphatase
,phosphate and calcium. Alkaline phosphatase may be as high as 3.5 times
normal at 4 weeks post injury with peak levels measured at 12 weeks. Smaller
volumes of ectopic bone (usually at the smaller joints) may not raise levels
as significantly(18).Although non-specific, serum alkaline phosphatase
may be the earliest ,least expensive test for early detection of HO. If
fractures are not present this is an excellent presumtive test (9). Serum
alkaline phosphatase elevation is accompanied by an increase in inorganic
phosphate and is preceded by a transient decrease in serum calcium(8).Other
researchers feel that these test are unreliable for screening(20).
RISK FACTORS:
Risk factors of HO include trauma, burns , neurologic injury, previous
HO (which may or may not have been resected ), surgery about the hips,
male sex(4),age over 60(4)and possibly genetic predisposition. Probable
risk factors include diffuse idiopathic skeletal hyperostosis(DISH), Paget's
disease(4). Forearm fractures in patients which are neurotraumatized or
burned have substantially increased risk of developing HO. The elbow is
the most frequently involved joint in burn patients. Burn-related HO was
more related to the severity of the burn. Burns over 20% of the body with
the majority of the burns being third degree(19). This may be evidence
of a humeral mediated response or systemic effect of trauma. Performing
hip soft tissue releases and spine surgery in children with cerebral palsy
concomitantly may increase the risk of HO(15). Genetic risk factors once
were thought to include those with positive HLA B18(18), HLA B27,HLADW7(9)
for neurogenic HO. However follow-up studies have not confirmed these findings.
Presently they have no predictive value(18).
Other risk factors include prior surgery for excision of HO, trochanteric
osteotomy , length of surgery,(9)and pressure sores near proximal joints.
INCIDENCE:
Incidence of Ho has been shown to occur generally in 10-20% of predisposed
patients(9). Reported rates vary with type of treatment center ; acute
care will see less incidence than rehabilitation units, since it is seen
later. Acetabular fractures treated with ORIF may have as high as 60% incidence
of HO however in many cases there may not be a limitation of motion and
many will improve function with time. Spinal cord injuries have 20-25%
incidence however 18-35% limit motion (4).Closed head injuries have 10-20%
incidence with 10% developing loss of motion . Incidence of HO in elbow
dislocations is about 3% but in fracture-dislocations it is 15-20 % (4).
Payne and DeLuca (10) reported 27%incidence of HO with selective rhizotomy
and subsequent femoral varus derotational osteotomy in spastic quadriplegics.
Ho was not noted in diplegics, nor in patients who did not undergo rhizotomy
during the same period. Patients with perthes disease who underwent adductor
releases had a incidence of 6% of HO.
CLASSIFICATION:
Classification of HO is well documented by Parkinson et al (22); DeLee(23);and
Brooker(24). These classifications were used to describe HO after total
hip arthroplasty. A classification developed by Krum and Miller(15) included
radiographic and clinical criteria. Grade I was considered when there was
no symptoms but radiographic evidence. Grade II was described as having
mild-moderate symptoms of pain, irritability, limited range of motion.
In grade III lesions hip function was severely limited. The radiographic
classification A included those lesions in which the width of heterotopic
ossification was less than half of the width of the femoral neck. With
B lesions the width of HO was equal to half of the width to the entire
width of the iplilateral femoral neck. Grade C heterotopic ossification
has lesions greater than width of the ipsilateral femoral neck. Classification
of HO about the elbow is described by Hastings and Graham(14). This three
part classification focuses on functional limitation with consideration
to the anatomic basis of HO distribution.
PROGNOSIS:
Prognosis for recovery of motion after HO is worse in patients that
have severe loss of ROM, severe persistent spasticity or poor neurologic
recovery. Elevated levels of serum alkaline phosphatase 1-2 years post
injury is a poor prognostic indicator . Minimal resorption of HO occurs
after it has matured however nearly full range of motion may return .
MANAGEMENT:
Non-surgical management of HO includes recognition of risk factors.
One should consider pharmacologic treatment. Agents used are diphosphonates
and NSAIDs. Etidronate disodium, Ethane Hydroxy diphosphonate prevent or
inhibit crystallization of hydrozyapatite, They do not interfere with formation
of osteoid or existing osteoid. Side effects include GI disturbances, osteomalacia
and rebound calcification of existing osteoid after treatment is discontinued.
Naraghi no longer recommends diphosphonates for prophylaxis against HO(4).
NSAIDS such as indomethicin, acetylsalicylic acid , and ibuprofen work
by inhibiting cycloxygenase which probably interrupts the synthesis of
PGE2, bone formation and fracture healing are suppressed. Efficacy has
been demonstrated in the hips of adults. Acetylsalicylic acid has been
reported to decrease progression in pediatric patients with HO following
brain injury. Most common side effects of NSAIDS are GI disturbances, and
interaction with other medicines , particularly anticoagulants.
Radiotherapy using a low-dose external beam radiation prevents cell
proliferation(25)/ Dosage is usually 700rads x1 dose or 1000 rads in 4
divided doses (26). There has been reports of sarcomas induced by radiation,
but not after receiving these recommended doses(27). Prophylaxtic post
surgical debridement of HO dosage is 700-800 rads within 48-72 hours after
surgery(14).
Physical therapy particularly stretching of spastic limbs has been postulated
as an etiology of HO however Wharton and Morgan reported in a study of
spinal cord injured patients , daily range of motion program improved range
of motion in such patients(28).Garland(9) recommended up to three manipulations
under anesthesia spaced one to two months apart for traumatic brain injured
patients with HO In traumatic HO , passive stretching of the joints may
lead to increase HO(4). Alteration of physical therapy should be made if
patient is diagnosed with HO . Forceful passive ROM should be avoided and
gentle ROM should be limited by the patients discomfort.
Surgical treatment should not be considered until the HO has matured
. Many researchers feel that 6 months after initial trauma (without neural
injuries) that HO can be resected. It should not be resected until it is
considered mature bone(13). A specific amount of time to delay surgical
resection of HO associated with with spinal cord injury is difficult to
predict. Surgery is usually delayed at least one year , but should be performed
at 1.5 to 2 years in young males with anklyosis or near ankylosis of the
hip, radiographic evidence of progression of HO longer than 6 months, greater
than moderate amounts of HO, severe spasticity, persistent elevation of
serum alkaline phosphatase, continued uptake in bone scanning, and poor
response to prophylactic medications. Procrastination longer than 2 years
allows development of complications such as intraarticular joint ankylosis
and fractures in the osteoporotic bone with initiation of joint motion
after resection (29).In traumatic brain injury patients the natural history
of neurologic recovery is the best index for surgical excision, recurrence,
and functional outcome(30). The majority of motor recovery occurs by 1.5
years -- those patients with rapid neurologic recovery may have HO resected
if the guidelines for monitoring the maturity of the bone are met(9).Surgery
is mainly indicated for limb positioning in the neurologically compromised
patient.
Techniques of surgery should be meticulous with adequate hemostasis,
evacuation of bone dust, interposition of muscle, fat, fascia or silastic(14).
More than one incision to decrease the dissection of soft tissue to reach
the HO may be beneficial(14). Complications of surgical resection include
recurrence, blood loss (this is especially true when HO has not matured,
infection, and morbidity.
DISCUSSION:
Heterotopic ossification is a well documented complication of spinal
cord injuries, surgical release of the spastic hip muscles, and trauma.
Local trauma is not a requisite of HO. Spinal fusion of cerebral palsy
patients may also be associated with HO at the hip area. Orthopedists ,
physical therapists , and pain management teams should be aware of this
complication when diagnosing and treating the cause of persistent post-op
pain. Recognition of risk factors may warrant prophylaxtic treatment.
REFERENCES:
- Geshickter C.F. JBJS 1938 No. 20 661-674
- Dejerine A, Ceillier A. Paraosteoarthropathies of paraplegic patients
by spinal cord lesion, Clin Orthop. 1991 263:3-12
- Damanski, M,: Heterotopic ossification in paraplegia, A clinical study.
JBJS 43B:286 1961
- Naraghi F, et al Review Heterotopic ossification. Orthopedics Feb.
1996 Vol 19 No2
- Urst, M.R. Mizulani H, TadagiK, et al A bovine low molecular weight
bone morphogenic protein fraction. Clinical Orthopedics 162-219 11982
- Fujimori Y, Nakamura T, Ijiri S, et as Heterotopic bone formation induced
by bone morphogenic protein in mice with collagen-induced arthritis, Biochem
Biophys Res Comm 186:1362 1992
- Huggins C.B. The formation of bone under the influence of epithelium
of the urinary tract Archives of Surgery 22: 377, 1931
- Ho SSW, Stern P.J., Bruno L.P. et al: Pharmacologic inhibition of prostaglandin
E2 and its effect on pathological new bone formation in a rat burn model.
Trans Orthop Res Soc 13-536, 1988
- Garland D.E. A clincal perspective on common forms of acquired heterotopic
ossification CORR No 263 Feb,1991
- Payne L.Z. DeLuca P.A. Heterotopic ossification after rhizotomy and
femoral osteotomy. JPO vol 13 no6 1993
- Reed M.H. ,et al Heterotopic ossification in children after iliopsoas
release. Canadian association of radiologists journal Vol43, No 3 June
1992
- Lee M, Alexander M.A. , Miller F. et al Postoperative Heterotopic ossification
in the child with cerebral palsy; three case reports Archives of physical
medicine and rehabilitation Vol 73, march 1992
- Khan F.A Bilateral ankylosis of the hips following heteropic ossification
of the ilio-ploas in a child. International orthopaedics : Springer-verlag
1992
- Hastings H. , Graham T. J. The classification and treatment of heterotopic
ossification about the elbow and forearm. Hand clinics Vol 109 No 3 August
1994
- Krum S.D. , Miller F. Heterotopic ossification after hip and spine
surgery in children with cerebral palsy. JPO vol 13 No. 6 1993
- Ainsworth S. R., Aulicino P.L. Chronic posterolateral dislocation of
the elbow in a child. Orthopedics
- Feb. 1993 Vol. 16 15 212-215.
- Thomas B, Amstutz H: Results of the administration of diphosphonate
for the previntion of heterotopic ossification after total hip arthroplasty.
JBJS 67-A: 400, 1985
- Garland D.E. ,Alday B, Venos K.G.: Heterotopic ossification and HLA
antigens, Archives of Physicas Med, Rehabilitation, 65:531, 1984
- Hoffer M M, Brody G,et al. Excision of heterotopic ossification about
elbows in patients with thermal injury. J Trauma 18:667 1978
- Mollan RAB. Serum alkaline phosphatase in heterotopic para-articular
ossification after total hip replacement, JBJS 1979 61B 432-434
- Hoppenfeld S. Zeide M.S. Orthopaedic dictionary 1994 J.B. Lipincott
- Parkinson et al Radiation therapy in the prevention of heterotopic
ossification after total hip arthroplasty. In Hip: proceedings of the tenth
annual scientific meeting of the hip society, St Louis, C.V. Mosby, 1982
- DeLee et al. Ectopic bone formation following lowfriction arthroplasty
of the hip. Clin orthop 1976;121:53-9
- Brooker AF et al Ectopic ossification after total hip replacement,
Incidence and a method of classification JBJS 1975: 55A 1629-32
- Tonna EA ,Cronkite EP autoradiographic studies ofcell proliferation
in the periosteum of intact and fractured femora of mice utilizing DNA
labeling with H3 thymidine Proc Soc Exp Bio Med 107:719, 1961
- Abrams RA, Simmons BP, Brown RA, et al: Treatment of posttraumatic
radioulnar synostosis with excision and low-dose radiation. J hand surg
18-A:703, 1993
- Brady LP, Jewett EL: a new treatment of radio-ulnar synostosis , South
Med J 43;507, 1960.
- WhartonGW,Morgan TH . Ankylosis in the paralyzed patient, JBJS 52-A
1970 ; 105-12
- Garland DE, Orwin JF : Resection of heterotopic bone in patients with
spinal cord injuries. Clin. orthop. 242:169, 1989
- Garland DE , Hanscom,DA , Keenan MA, Smith C and Moore T, Resection
of heterotopic ossification in the adult with head trauma. JBJS 67A:1261
1985.
 .
On 9\95 patient underwent bilateral release of iliopsoas, gracilis, adductor
longus, distal hamstrings and right tensor facia lata release. Four weeks
later the patient still required valium and analgesics due to pain and
muscle spasms. At that time a firm mass was palpated in the left groin.
On 11\95 The patient was still having pain
.
On 9\95 patient underwent bilateral release of iliopsoas, gracilis, adductor
longus, distal hamstrings and right tensor facia lata release. Four weeks
later the patient still required valium and analgesics due to pain and
muscle spasms. At that time a firm mass was palpated in the left groin.
On 11\95 The patient was still having pain  .
Four months post-op the patient was able to start aquatic therapy. Seven
months post-op the patient had increased range of motion with less pain.
.
Four months post-op the patient was able to start aquatic therapy. Seven
months post-op the patient had increased range of motion with less pain.